Microthrix Bulking
in Summer
by Deborah Lee, Microbiologist
Why do we care about Microthrix filaments?
Usually, Microthrix parvicella is associated with foaming problems in wastewater treatment. Sometimes we have observed high levels of what looks like M. parvicella in a sample of mixed liquor, and though the plant may be experiencing bulking, they do not have any foam. Even more confusing is when this occurs in summer, since M. parvicella is associated with cold temperatures in winter. It turns out that Microthrix can cause bulking, with or without foaming (Seviour et al., 2008). We often see this because these filaments grow outward from the floc faster than the floc-formers can grow, which helps them gain access to substrates in the bulk liquid (Martins et al., 2004). This helps filaments cope with feast and famine periods, and in the case of Microthrix, lets them rapidly assimilate substrates and store them intracellularly as PHA, lipids, or other compounds (Seviour et al., 2008).

The typical M. parvicella was first described by van Veen in 1973, after which several isolates were cultured, but only a few were maintained in pure culture (Rossetti et al., 2005). Recently it was argued that Microthrix parvicella is not a taxonomically valid name due to a lack of detailed phenotypic characterization. However, the name is retained, and the organism has been elevated to Candidatus status because it is the vernacular name for this filament and this name has been used throughout the wastewater industry (Rossetti et al., 2005). The name retention also applies to the observation of a filament with slightly thinner trichomes but was otherwise indistinguishable from M. parvicella. The two filaments could be distinguished by 16S rRNA sequences and this emphasizes the need for precise FISH identification (Seviour et al., 2008; Tandoi et al., 2017) in research. This similar filament was found to grow on long-chain fatty acids (LCFA) and had exoenzymes to break down lipids similar to M. parvicella, but could grow at temperatures >30°C, but not at 10°C (Levantesi et al., 2006). This filament was named Microthrix calida (Levantesi et al., 2006). Microthrix calida can also cause bulking but, unlike M. parvicella, it does not cause foaming (Cosenza et al., 2013).
Morphology
Microthrix parvicella is a filamentous bacterium that forms long, thin filaments with diameters of 0.6-0.8 µm (Rossetti et al., 2005). It is unbranched and does not form sheaths; however, the long filaments tend to form coils (Rossetti et al., 2005). It has a strong Gram-positive reaction (Rossetti et al., 2005) and often has many polyphosphate (poly-P) granules that are easily visible after Neisser staining (van Veen, 1973). This organism is known to cause foaming and even inactive cells may cause or maintain foam (Fan et al., 2017). This organism is also known to cause bulking in activated sludge.
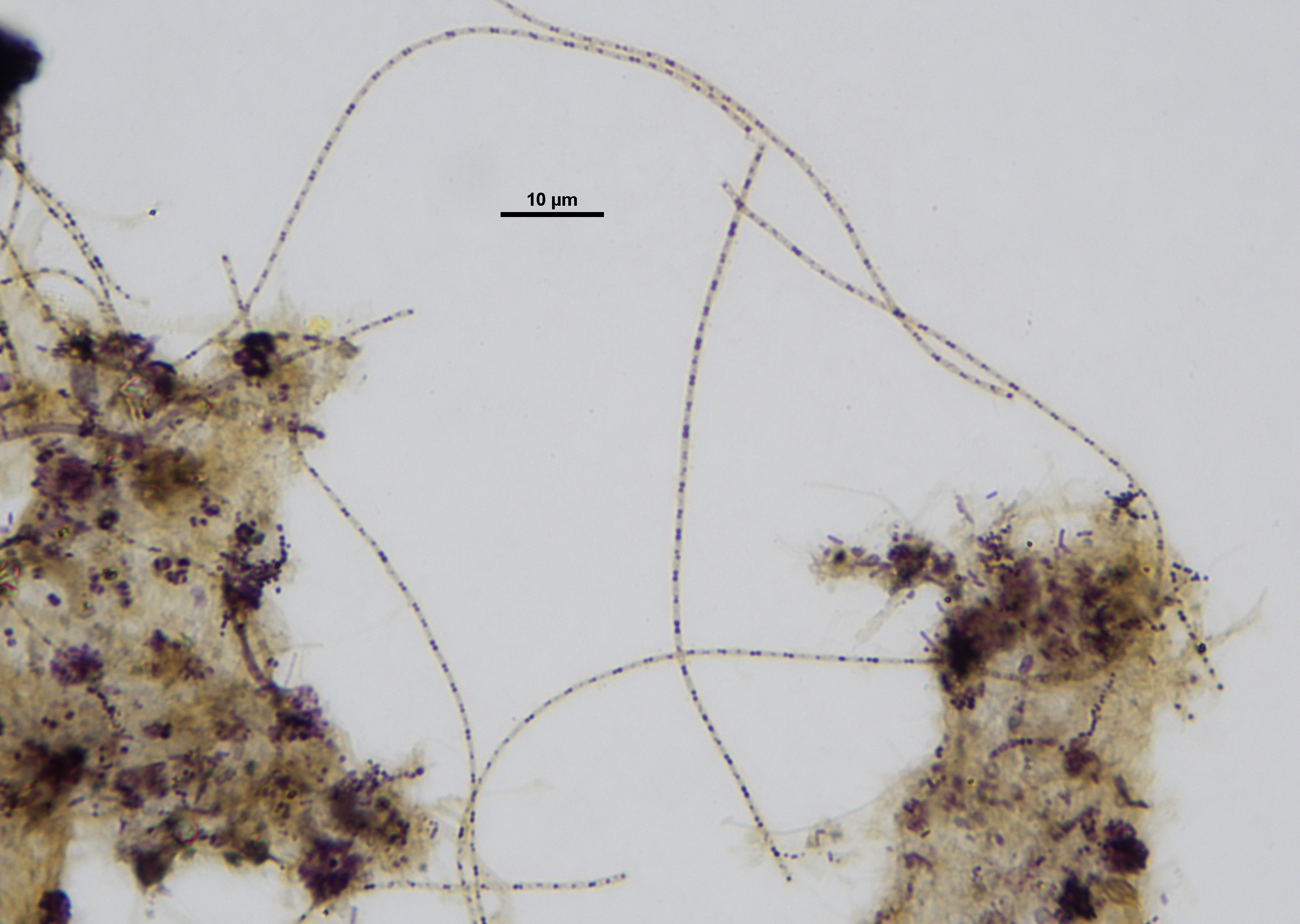
Microthrix calida is a filamentous bacterium that forms long, thin filaments with diameters of 0.3-0.7µm (Levantesi et al., 2006). This smaller filament diameter is not always a reliable distinguishing characteristic for differentiation (Levantesi et al., 2006). It is similar in morphology to Microthrix parvicella; however, it is reported to have a weaker Gram-positive reaction (Levantesi et al., 2006). It is not known to cause foaming, but it can cause bulking in activated sludge (Cosenza et al., 2013). A further difference from M. parvicella, is M. calida grows at higher temperatures—up to 36.5°C (Levantesi et al., 2006; Fan et al., 2017). M. calida has so far been found in some industrial plants and found to generally co-exist with M. parvicella (Levantesi et al., 2006).
References
Cosenza, A., Di Bella, G., Mannina, G., & Torregrossa, M. (2013). The role of EPS in fouling and foaming phenomena for a membrane bioreactor. Bioresource Technology, 147: 184-192.
Fan, N., Qi, R., Rossetti, S., Tandoi, V., Gao, Y., & Yang, M. (2017). Factors affecting the growth of Microthrix parvicella: Batch tests using bulking sludge as seed sludge. Science of the Total Environment, 609, 1192-1199.
Levantesi, C., Rossetti, S., Thelen, K., Kragelund, C., Krooneman, J., Eikelboom, D., Nielsen, P. H., & Tandoi, V. (2006). Phylogeny, physiology and distribution of ‘Candidatus Microthrix calida’, a new Microthrix species isolated from industrial activated sludge wastewater treatment plants. Environmental Microbiology, 8(9), 1552-1563.
Martins, A. M. P., Pagilla, K., Heijnen, J. J., & van Loosdrecht, M. C. M. (2004). Filamentous bulking sludge – a critical review. Water Research 38, 793-817.
Nierychlo, M., Singleton, C. M., Petriglieri, F., Thomsen, L., Petersen, J. F., Peces, M., Kondrotaite, Z., Dueholm, M. S., & Nielsen, P. H. (2021). Low Global Diversity of Candidatus Microthrix, a Troublesome Filamentous Organism in Full-Scale WWTPs. Frontiers in Microbiology, 12, Article 690251.
Rossetti, S., Tomei, M. C., Nielsen, P. H., & Tandoi, V. (2005). “Microthrix parvicella”, a filamentous bacterium causing bulking and foaming in activated sludge systems: a review of current knowledge. FEMS Microbiology Reviews, 29, 49-64.
Seviour, R. J., Kragelund, C., Kong, Y., Eales, K., Nielsen, J. L., & Nielsen, P. H. (2008). Ecophysiology of the Actinobacteria in activated sludge systems. Antonie van Leeuwenhoek 94, 21-33.
Slijkhuis, H. (1983). Microthrix parvicella, a Filamentous Bacterium Isolated from Activated Sludge: Cultivation in a Chemically Defined Medium. Applied and Environmental Microbiology, 46(4), 832-839.
Slijkhuis, H., van Groenestijn, J. W., & Kylstra, D. J. (1984). Microthrix parvicella, a Filamentous Bacterium from Activated Sludge: Growth on Tween 80 as Carbon and Energy Source. Journal of General Microbiology, 130, 2035-2042.
Tandoi, V., Majone, M., & Rossetti, S. (2017). Chapter 5 Bulking and foaming control methods. In Activated Sludge Separation Problems: Theory, Control Measures, Practical Experiences. essay, IWA Publishing.
Van Veen, W. L. (1973). Bacteriology of activated sludge, in particular the filamentous bacteria. Antonie van Leewenhoek, 39, 189-205.

About the Author
Deborah Lee is a Senior Microbiologist at Aquafix with 12 years of experience studying nitrifying bacteria. She works with our wastewater clients to understand nitrifier populations and how to improve them.
