
Everything You Need To Know
About Aerobic Denitrifiers
by Deborah Lee,
Aquafix Senior Microbiologist & Nitrifier Expert
What are Aerobic Denitrifiers?
Aerobic denitrification is the simultaneous use of both oxygen and nitrate (NO3–) as the terminal electron acceptor. A terminal electron acceptor is a compound that gets reduced in the reaction by receiving electrons. Some bacteria are able to denitrify under aerobic conditions (e.g. Paracoccus denitrificans), while conventional denitrifiers can grow under aerobic conditions, but denitrify only in the absence of oxygen (e.g. Pseudomonas aeruginosa). The aerobic denitrifying bacteria are mainly in the phylum Proteobacteria. Denitrification, aerobic or anoxic, depends on the use of organic carbon compounds, so bacteria that perform denitrification are heterotrophic organisms. Autotrophic denitrification, using sulfur, does exist, however, we will just look at the heterotrophs here. Heterotrophic denitrification, aerobic or facultative, uses nitrate to generate ATP and eventually produces nitrogen gas as an end product (see formula below).
2 NO3– + 10 e– + 12 H+ → N2 (g) + 6 H2O
How does Aerobic Denitrification Compare to Facultative Denitrification ?
Denitrification is the process to remove nitrate and nitrite resulting in the release of nitrogen gas (N2). It was conventionally believed that denitrification would not occur in the presence of oxygen. Reasons for this mainly center on oxygen yielding more energy so it was not advantageous to perform denitrification if oxygen was available. It has since been found that aerobic denitrification does occur in many different bacteria even if this results in slightly lower levels of cell reproduction compared to pure aerobic respiration.
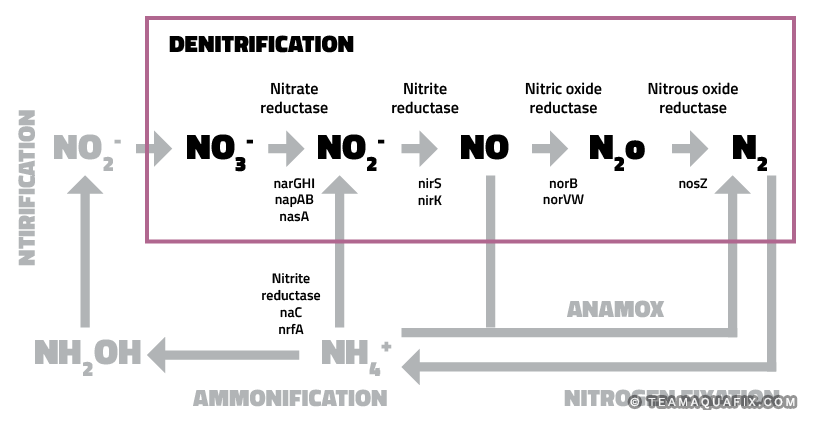
Figure 1: Inorganic nitrogen pathways, picture from: Alvarez et al., 2014
It’s uncertain if there is really an advantage to organisms to be able to perform denitrification in the presence of oxygen. However, it is possible that this is a viable adaptation to rapid changes in environmental oxygenlevels when organic carbon compounds are available. Conventional facultative denitrifying bacteria require very low oxygen concentrations since the presence of oxygen in these bacteria will shut off the denitrification enzyme production. In most WWTP, conventional denitrifiers would be found near the beginning of the plant where the waste first enters in an anoxic zone or anoxic basin, while nitrifiers would be in the subsequent downstream aerated basin. This means nitrate is generated in the plant from nitrification (ammonia oxidation and nitrite oxidation) and may leave the plant in the final effluent.

Where do Aerobic Denitrifiers Live ?
Denitrifying bacteria are commonly found in soil and aqueous environments. They are important in removing nitrogen from industrial and municipal wastewater. These types of environments with fluctuating oxygen concentrations will favor bacteria that have constitutive (always on) denitrification enzymes. This way the aerobic denitrifying bacteria gain energy from using both oxygen and nitrate/nitrite as electron acceptors while taking less time to turn each individual pathway on or off. The tradeoff for such bacteria is that they do not see as high of an increase in cell biomass as in entirely aerobic environments.
What do Aerobic Denitrifiers Need to Grow
Aerobic denitrifying bacteria prefer dissolved oxygen (DO) in the range of 3-5 mg/L. The activity of denitrification enzymes increases significantly when the DO concentration decreases to a certain value, which is specific for the species of aerobic denitrifier. That value is called the oxygen threshold. Some species of denitrifiers are known to function at DO as high as 10 mg/L. The DO is often affected by temperature. Temperature also determines how fast molecules move and so how fast reactions occur. At higher temperatures, molecules move faster and reactions tend to be faster while at lower temperatures everything moves slower and reactions will take longer. The typically studied aerobic denitrifiers generally prefer temperatures around 25-37oC. In many cases when the temperature drops below 10oC, aerobic denitrification is seriously reduced, and significant nitrite accumulation may occur. However, there are cold-tolerant aerobic denitrifiers that can function even at temperatures as low as 70oC and if cold temperatures persist for a long time these aerobic denitrifiers can become the dominant population of aerobic denitrifiers. During denitrification, the pH will increase as a result of alkali formation. Aerobic denitrifiers generally prefer pH 7, but will function in pH 6.5-8.0, but denitrification tends to be reduced at pH 5. Denitrification depends on having organic carbon compounds such as glucose, acetate, succinate, citrate, or other simple sugars. The optimal carbon-to-nitrogen ratio (C:N) depends on the species of aerobic denitrifier but is generally around 5-10. This is a higher carbon requirement than conventional facultative denitrification.
Why do Aerobic Denitrifying Organisms Exist?
It seems that aerobic denitrification is the rule rather than the exception since there are many different aerobic denitrifiers found in various environments. One of the explanations for aerobic denitrification prevalence is that it is a way for bacterial cells to dispose of excess reducing equivalents. It may also be that aerobic denitrifiers evolved as denitrification specialists but lost the ability to shut off denitrification in the presence of oxygen. For whatever reason they developed, aerobic denitrifiers may offer the potential to wastewater treatment plants to eliminate the need for separate tanks (for denitrification and nitrification) and reduce sludge yield.

About the Author
Deborah Lee is a Senior Microbiologist at Aquafix with 11 years of experience studying nitrifying bacteria. Deborah works with our wastewater clients to understand nitrifier populations and how to improve them. Deborah also oversees the production of nitrifying bacteria at Aquafix.


