All About Essential Nutrients
Complete Nitrogen Guide
How It Keeps Your Plant Healthy
by Saylor Gilbert, Research Scientist

Welcome to the first installment of our three-part blog series, where we delve into the relationship between aerobic wastewater treatment and the essential nutrients: carbon, nitrogen, and phosphorus. Many operators and engineers know the textbook ratios – ranging from 100:5:1 to 100:10:1 – and understand that carbon, nitrogen, and phosphorus originate from compounds targeted for removal in aerobic wastewater treatment. However, this series aims to go deeper, offering knowledge into why these nutrients are crucial and how they are effectively removed during treatment. In today’s post we will focus on nitrogen – examining the various forms it takes, its removal processes, and vital role as a nutrient.
Nitrogen entering a wastewater system primarily exists in two forms: inorganic and organic nitrogen compounds. Inorganic nitrogen includes ammonia, ammonium, nitrite, and nitrate, along with gaseous forms like nitric oxide, nitrous oxide, and atmospheric nitrogen. These gases are typically produced during denitrification, a process we’ll explore later in the blog, but are usually absent from wastewater influent. Organic nitrogen compounds include proteins, amino acids, urea, uric acid, and various other amino acids. Simply put, organic nitrogen tends to originate from organic sources, characterized by their complex structures with many carbon-hydrogen bonds, while inorganic nitrogen

is simpler and lacks these bonds. These complex organic nitrogen compounds can undergo hydrolysis, breaking down into simpler forms and eventually being converted to ammonia. This conversion is a crucial part of how nitrogen is removed from influent.
Nitrogen removal in aerobic wastewater treatment occurs through two key processes: conversion and assimilation. In simple terms, conversion involves transforming nitrogen into more and more simplistic forms, while assimilation refers to the uptake of nitrogen by microorganisms for the production of biomass. Both processes ultimately lead to nitrogen being removed from the system, either as nitrogen gas escaping into the atmosphere or as part of the biomass that is extracted as waste activated sludge.
When nitrogen enters the treatment plant, it comes in as either inorganic or organic compounds, as we discussed earlier. Some of this nitrogen is immediately assimilated by heterotrophic bacteria. The nitrogen that isn’t assimilated immediately undergoes a series of conversions. Organic and inorganic nitrogen compounds are ultimately broken down into ammonia. After this occurs, autotrophic nitrifying bacteria take over: ammonia-oxidizing bacteria (AOB) convert some of the ammonia into nitrite, and nitrite-oxidizing bacteria (NOB) further convert the nitrite into nitrate. In systems equipped with denitrification capabilities, this nitrate is converted by denitrifying bacteria into gaseous forms, which bubble out of the system. In systems without capacity for denitrification, the remaining ammonia and nitrate are instead assimilated.
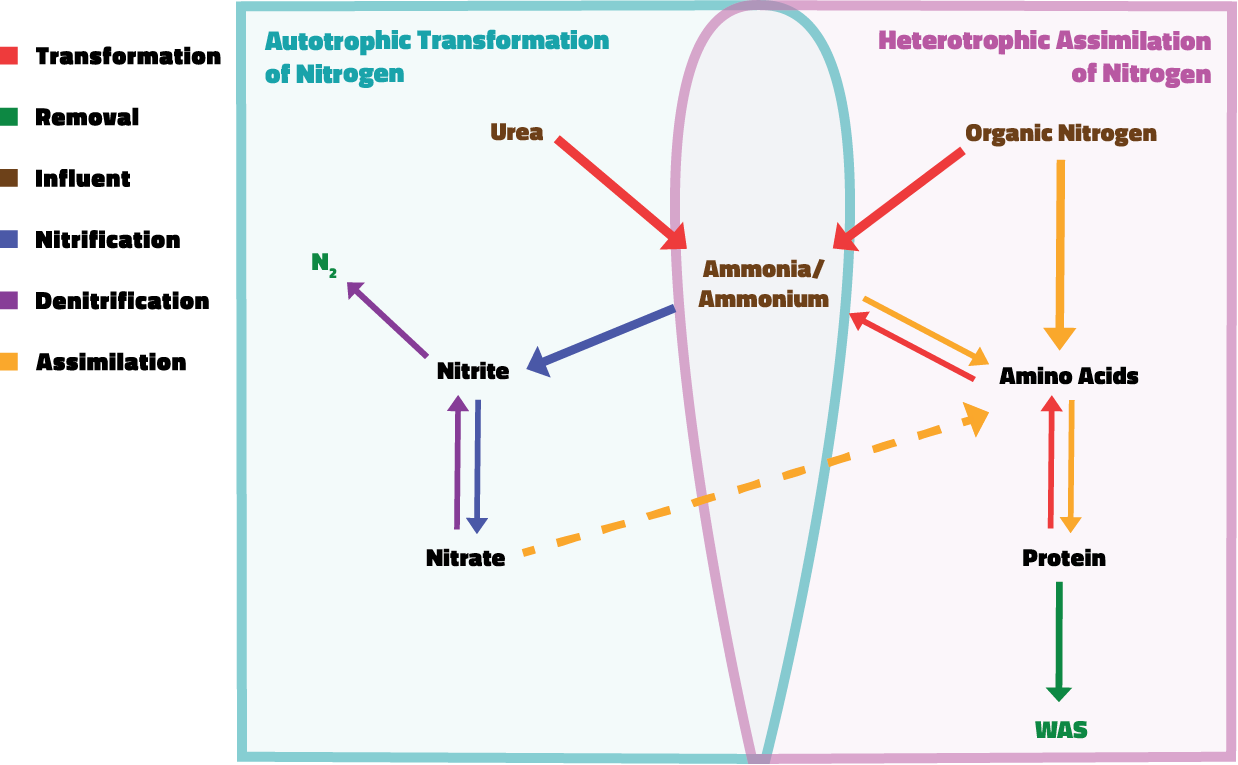
This blend of conversion and assimilation ensures that nitrogen is effectively removed from the wastewater, either through incorporation into microbial biomass or as nitrogen gas. But why is nitrogen so crucial for bacterial assimilation and biomass production?
The reason nitrogen is so crucial in wastewater treatment boils down to proteins. Proteins are the fundamental building blocks of all living organisms, which includes the bacteria that constitute the majority of the biomass within a wastewater treatment facility. These proteins are composed of amino acids, which all contain amine groups that require nitrogen. The organic nitrogen compounds that were assimilated early in the nitrogen removal process can be directly converted into amino acids and used for protein production, bypassing the need for complex synthesis. However, other forms of nitrogen must undergo intricate processes which involve converting ammonia into primary amines then into secondary and tertiary amines. These amines are used to synthesize amino acids, which are ultimately used to build proteins. While this assimilation process is crucial for the removal of inorganic nitrogen, it is inefficient and requires complex enzymes and cellular machinery.
The key takeaway is that nitrogen removal is a somewhat cyclical process. Healthy biomass requires quality proteins, quality proteins require sufficient nitrogen, and nitrogen assimilation relies on healthy biomass. If there’s a disruption at any point in this cycle, the entire process is compromised, and nitrogen removal is harmed. In a system that is deficient in nitrogen the process falters. Urea is a common nitrogen supplement, but it is an organic nitrogen source that isn’t directly assimilated; instead, it is hydrolyzed into ammonia. As discussed, protein synthesis from ammonia is much more complex than protein synthesis from other organic nitrogen compounds. For this reason, it is often better to supplement nitrogen, at least in part, with complex organic nitrogen (such as those found in Accelerator 7). This provides organisms with an easily assimilable nitrogen source, improving their ability to then assimilate other less efficient nitrogen sources that still need to be removed.
While understanding nitrogen removal and the nitrogen needs of your wastewater system may not be strictly required to operate a wastewater facility, it does allow for more dynamic and informed troubleshooting. It helps operators and engineers anticipate and avoid potential upsets, leading to better overall system performance. If you have any questions about this blog or Aquafix services and products, including nitrogen supplementation, please feel free to reach out. Thank you for your time.
Nitrogen Solutions
Accelerator 7
- Bioavailable nitrogen supplement
- Reduce low nitrogen filaments
- Great for industries with uniform waste streams
SmartBOD
- Balanced blend of nutrients to promote flocculation and improve settling
- Better performance compared to dog food and glycerin
- Great for systems with low or intermittent loading
- Lowers effluent BOD, TSS, ammonia, and phosphorous while reducing bulking

About the Author
Saylor Gilbert is a Research Scientist for Aquafix and holds a bachelor’s in microbiology. His expert knowledge of toxicity and F.O.G. allows us to push our products forward and continue to provide our customers with top-tier wastewater solutions.



